How a tiny cellular “motor” helps protect our mitochondrial DNA
02.07.2025
 |
|
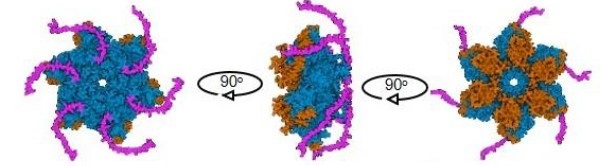
Twinkle consists of an ancestral N-terminal primase domain and a C-terminal helicase domain (magenta). Credit: Borja Ibarra. |
- Researchers at IMDEA Nanociencia observe the real time kinetics of Twinkle –a protein critical to copying and preserving mitochondrial DNA.
- The study reveals that the activity of Twinkle is strongly autoregulated by specific domains within the protein.
| Tweet |
Madrid, July 2nd, 2025. In a step toward understanding how our cells maintain healthy energy production, researchers at IMDEA Nanociencia have revealed the inner workings of “Twinkle,” a protein critical to copying and preserving mitochondrial DNA. The study, combining biochemical and single-molecule techniques, shows how Twinkle operates in real time and how its activity is tightly regulated—insights that could have broad implications for understanding mitochondrial diseases and aging.
Mitochondria, often called the powerhouses of the cell, depend on their own DNA to function properly. Twinkle is the helicase—a type of molecular motor—that unwinds this DNA so it can be copied accurately. The new research shows that Twinkle doesn’t simply power forward; instead, it carefully scans double-stranded DNA to locate the precise site where it needs to work. Once there, specific interactions with DNA and other regulatory parts of the protein slow it down, allowing for greater control and accuracy.
Key to this regulation are two parts of the Twinkle protein: the Zinc-binding domain (ZBD) and the C-terminal tail. These regions act like built-in brakes, adjusting the speed of the helicase depending on conditions. Interestingly, when another protein called mtSSB binds to the DNA, it seems to override the braking effect, allowing Twinkle to move more freely. The study also found that the same components that control unwinding also affect how the helicase rewinds DNA if it stalls—suggesting a finely tuned, self-regulating mechanism.
This research opens the door for even more detailed studies of Twinkle’s behavior in the context of the full mitochondrial DNA replication machinery. Understanding these mechanisms is vital, as defects in mitochondrial DNA replication are linked to a range of mitochondrial disorders, which can affect muscles, nerves, and energy levels.
This work is a collaboration among scientist at the Madrid Institute for Advanced Studies in Nanoscience (IMDEA Nanociencia), the University of North Florida, the Spanish National Cancer Research Centre (CNIO), and the National Centre for Biotechnology (CNB). It is partially funded by the accreditation Excellence Severo Ochoa awarded to IMDEA Nanociencia (CEX2020-001039-S).
Glossary:
- Twinkle: A helicase protein found in human mitochondria that is essential for unwinding mitochondrial DNA during replication.
- Mitochondrial Replisome: The full set of proteins, including Twinkle, that work together to replicate mitochondrial DNA inside cells.
Reference:
Ismael Plaza et al. Autoregulation of the real-time kinetics of the human mitochondrial replicative helicase. Nature Communications (2025). DOI: 10.1038/s41467-025-60289-0
![]() Link to IMDEA Nanociencia Repository: https://hdl.handle.net/20.500.12614/4018
Link to IMDEA Nanociencia Repository: https://hdl.handle.net/20.500.12614/4018
Contact:
Borja Ibarra
borja.ibarra [at] imdea.org
https://nanociencia.imdea.org/molecular-motors-manipulations-lab/group-home
Bluesky: @3M_Lab.bsky.social
Oficina de Divulgación y Comunicación en IMDEA Nanociencia
divulgacion.nanociencia [at]imdea.org![]()
![]()
![]()
![]()
![]()
Fuente: IMDEA Nanociencia.
IMDEA Nanociencia Institute is a young interdisciplinary research Centre in Madrid (Spain) dedicated to the exploration of nanoscience and the development of applications of nanotechnology in connection with innovative industries.